Alkaline and Ionized Water Explained ?
First: Let's get the basics correct. You will hear from people who have no knowledge of what pH is and the measure of pH.
Source: pH is a measure of the hydrogen ion concentration of a solution (Water in this case). Solutions with a high concentration of hydrogen ions have a low pH and solutions with a low concentrations of H+ ions have a high pH.
From the Source: table you will notice a few relationships:
- A difference of one (1) pH unit (ie from pH 2 to pH 3) is a ten fold (10X) difference in H+ ion concentration.
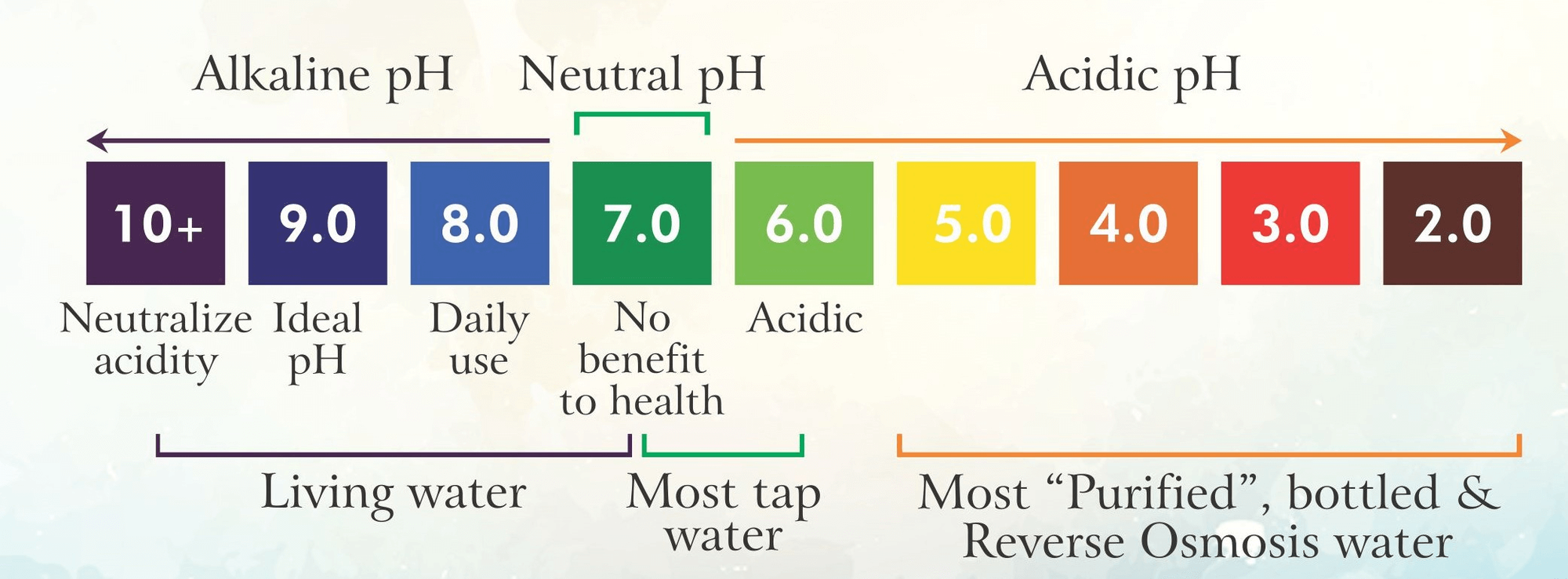
- Pure water usually is and should have a pH of 7.0
- Solutions with a pH below 7.0 are termed acidic and solutions with a pH above 7.0 are termed basic
Second: Walk into a convenience store and go to the drink cooler. You'll probably see an array of choices. Natural spring water is much higher priced than generic brands, but it also boasts to be from natural springs. Natural springs bottled water is usually marketed as alkaline water.
 |
|
ALKALINE WATER
Water that is slightly basic contains basic minerals such as calcium, magnesium, or bicarbonate. These compounds bind to hydrogen ions in solution, making the water increase in pH.
So, how do we get alkaline water if normal tap water is at about neutral pH?
Natural alkaline water sources are usually springs, or a reservoir of natural water underneath the earth's surface. The rock structures holding the water may have basic minerals, such as calcium or limestone, that leak into the water, increasing the pH.
Some companies add minerals to the water to make it Alkaline. Water can also be ionized, meaning it is broken up into hydrogen ions and H+ (hydroxide ions). The hydrogen ions are bound by minerals in the water, which makes the water Alkaline.
However, not all water has these minerals. Tap water will have a lower pH, because there are no minerals attached to the hydrogen ions.
INTERESTED IN THE PRODUCT - LEARN MORE | |
|---|---|
AVAILABLE IN | |
MALAYSIA&OUR ONLINE STORE | INDIA&OUR ONLINE STORE |
 |  |
MILDLY ALKALINE IONIZED WATER
Mildly alkaline water (pH 8-10) is marketed as healthy drinking water.1 It is produced from water ionizers via electrolysis.2 Alkaline water containing molecular hydrogen H+ is produced at the negative electrode (cathode).3 There are many names given to this water including: alkaline water,4 ionized water,5 alkali ion water,6 cathodic water,7 electrolyzed reduced water,9 and many more.
Electrolyzed reduced water (ERW) is the most common term in the scientific literature.1 It is called “electrolyzed” because the water has undergone electrolysis and is called “reduced” because the water at the cathode has been reduced to hydrogen gas and hydroxides.
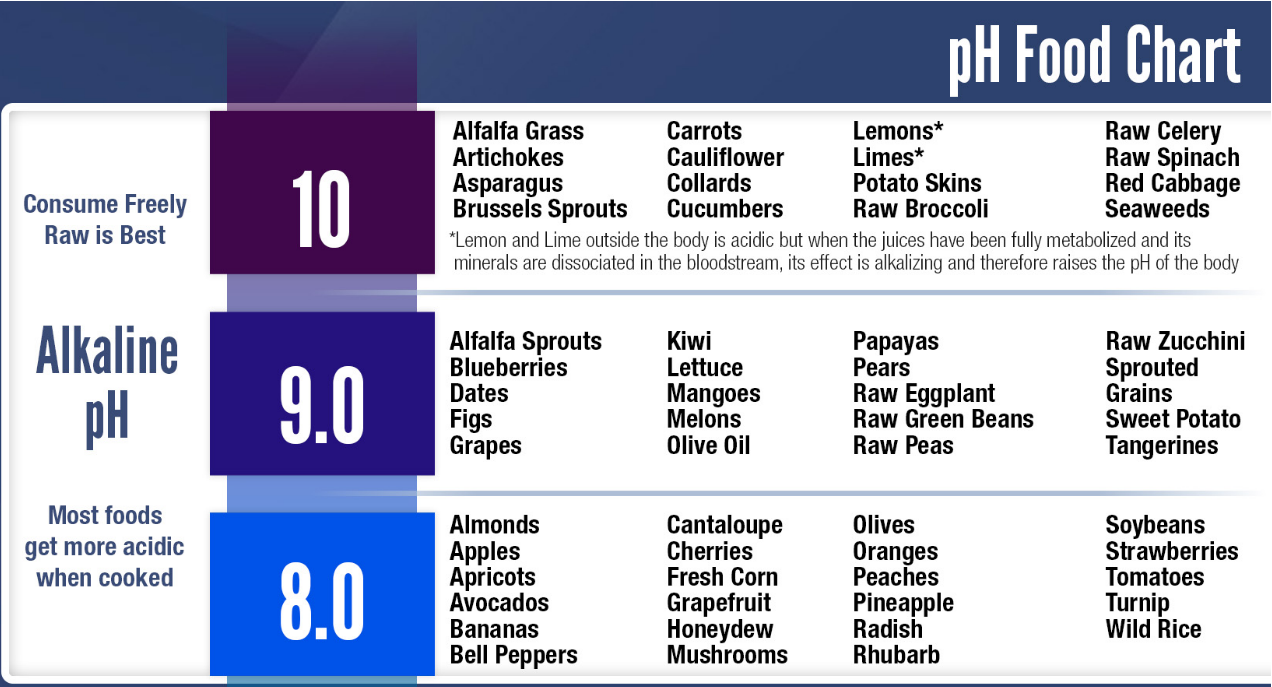
Talking about Alkaline: Some Fruits & Dry fruits and even vegetables are naturally Alkaline
Nano Filtration retains essential minerals (Potassium, Calcium and Magnesium) naturally available in water and makes water Alkaline.
Nano Filtration is an effective water filtration method which employs a combination of nanofiltration and ionization. Nanofiltration has a filtration pore size of around 0.001 microns. It effectively removes all contaminants like bacteria, protozoa and viruses as well as most natural organic matter, especially divalent ions, therefore softening water
| ||||||||||||||||||||||||||||||||||||
A study published by the World Health Organization cautions against drinking acidic or demineralized water (meaning water with no mineral content)
In most countries use of Reverse Osmosis (RO) purifiers are very dominant and commonly used and marketed as the best purified water.
Some major brands remineralizes the RO water using mineral filter / port/cartridge and some even use chemicals
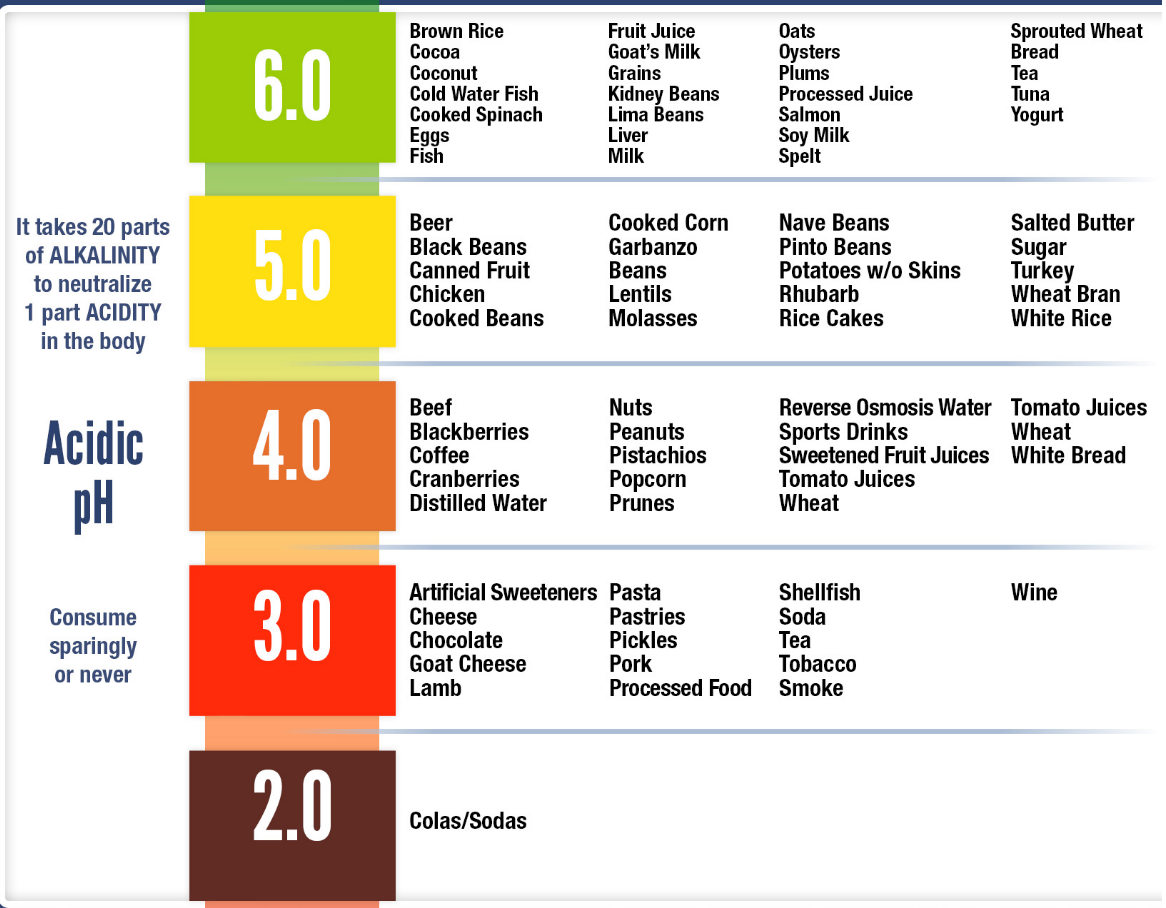
Here's a take away for you to understand how acidic our body becomes with the food and drink we consume on a daily basis.